Abstract
Background: Patients with relapsed or refractory (r/r) acute myeloid leukemia (AML) have limited treatment options. Outside of clinical trials and in the absence of a targetable mutation, there is no consensus on an optimal treatment regimen. Venetoclax (Ven), combined with hypomethylating agents (HMA) such as azacitadine (Aza) or decitabine (Dec), is approved as frontline treatment for elderly or unfit patients with AML. HMA/Ven is also being used frequently in the salvage setting. However, outcomes of HMA/Ven compared to IC for patients with r/r AML are largely unknown.
Methods: We conducted a retrospective study to compare outcomes of adult patients (>18y) with r/r AML treated with either HMA/Ven or IC at the University of Alabama at Birmingham. Patients treated with HMA/Ven were matched in a 1:1 ratio to patients receiving IC following a hierarchal algorithm based on age, ELN risk stratification, time to relapse (primary refractory/<6m vs. ≥6m) and line of therapy. Treatment with HMA consisted of either Aza 75mg/m 2 for 7 days or Dec 20mg/m 2 for 5 days. Venetoclax was administered at an effective dose of 400mg daily for 21-28 days per cycle (dose adjusted for concomitant azole use).
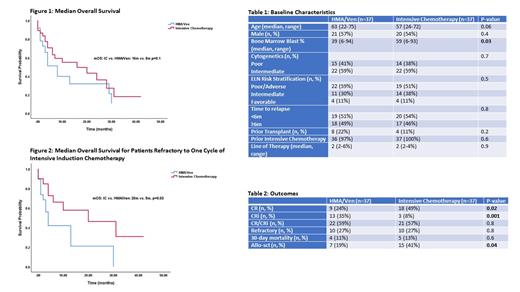
Results: There were 74 patients included in the analysis (HMA/Ven=37, IC=37). The baseline characteristics (Table 1) were well balanced with the exception of increased bone marrow blast percentage in the IC arm (59% vs. 39%, p=0.03). The median age of the IC and HMA/Ven groups was 56y (24-72y) and 63y (22-75y), respectively (p=0.06).
Regimens in the IC arm included FLAG (n=16, 43%), FLAG-ida (n=11, 30%), CLAG-M (n=5, 14%), 7+3 (n=3, 8%), HiDAC (n=1, 2.5%) and MEC (n=1, 2.5%). In the HMA/Ven arm, 21 patients (57%) received Aza and 16 (43%) received Dec.
The rate of complete remission (CR) was higher for IC (49% vs. 24%, p=0.02) whereas rate of CR with incomplete count recovery (CRi) was higher for HMA/Ven (35% vs. 8%, p=0.001) (Table 2). There was no difference in composite CR (CR+CRi) rates between the two arms (IC 57% vs. 59% HMA/Ven, p=0.8). Additionally, there was no difference in rate of refractory disease (27% vs. 27%, p=0.9) or 30-day mortality (IC 13% vs 11% HMA/Ven, p=0.6) between the two arms. More patients in the IC arm (41%), compared to the HMA/Ven (19%) arm proceeded to allogeneic stem cell transplantation (allo-sct) (p=0.04).
The median overall survival (mOS) for the IC arm was 16m, compared to 8m for the HMA/Ven arm (p=0.1) (Figure 1). The mOS for patients with primary refractory/<6m relapse from remission was 10m for IC and 6m for HMA/Ven (p=0.4). The mOS for patients refractory to one cycle of intensive induction (7+3 in approximately 90% cases in both arms) was 20m for the IC arm and 5m for the HMA/Ven arm (p=0.03) (Figure 2). The mOS for patients relapsing ≥6m from remission was 15m for IC and 9m for HMA/Ven (p=0.5). There was no difference in survival based on age, ELN risk stratification or cytogenetics.
Conclusion: Overall, there appears to be no significant difference in outcomes between IC and HMA/Ven for patients with r/r AML. Higher CR rates as well as ability to proceed to allo-sct are observed with IC. For patients refractory to the first cycle of intensive induction chemotherapy, a significant survival benefit was observed in those receiving IC compared to HMA/Ven. A second round of induction, preferably with a high-dose cytarabine based regimen, may provide better long term outcomes for these patients.
Vachhani: CTI BioPharma Corp: Consultancy; Abbvie: Consultancy; Agios: Consultancy; Blueprint Medicines: Consultancy; Pfizer: Consultancy; Seattle Genetics: Research Funding; Astellas Pharma: Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Novartis: Consultancy; O'Neal Comprehensive Cancer Center, University of Alabama at Birmingham: Current Employment; Jazz Pharmaceuticals: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal